Here is the link to the study.
The researchers, from the University of Cambridge, say their solar-powered reactor could be used to make fuel to power cars and planes, or the many chemicals and pharmaceuticals products we rely on. It could also be used to generate fuel in remote or off-grid locations.
Unlike most carbon capture technologies, the reactor developed by the Cambridge researchers does not require fossil-fuel-based power, or the transport and storage of carbon dioxide, but instead converts atmospheric CO2 into something useful using sunlight. The results are reported in the journal Nature Energy.
Carbon Capture and Storage (CCS) has been touted as a possible solution to the climate crisis, and has recently received £22bn in funding from the UK government. However, CCS is energy-intensive and there are concerns about the long-term safety of storing pressurised CO2 deep underground, although safety studies are currently being carried out.

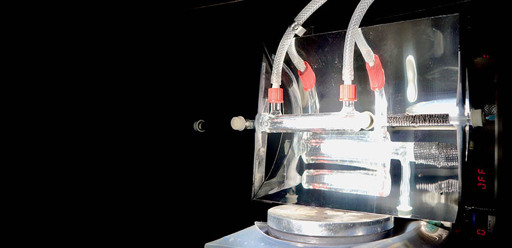
It’s making syngas (mix of hydrogen and CO).
So it begs for a next step. Syngas on its own is not a practical fuel. Unlike methane, it can’t be liquefied (hydrogen does not liqefy under economically feasible conditions). Due to free hydrogen, it makes metals brittle. Due to CO, it’s poisonous. And like most fuels, it’s flammable - I think we can’t blame a fuel for that. :)
The next step is hydrogenation of carbon monoxide. I’ve browsed literature and read my fair share via Sci-Hub, and this step tends to have various issues: reactivity, selectivity, catalyst cost and catalyst lifetime.
Reactivity: often, you have to raise either the pressure or the temperature to levels which complicate industrial production. Directly reacting CO2 with H2 faces those issues, but the catalyst (Cu + ZnO) is cheap.
Selectivity: suppose you want to get methanol, the simplest alcohol. Unfortunately your catalyst gives you a mix of methane, methanol, ethane, ethanol and buthanol. To build an industrial process, you need an extra step to separate them. If you get too much byproducts, your fuel production plant could become considerably bigger and more costly. So you definitely want good selectivity.
Catalyst lifetime: suppose that 1 kg of catalyst manages to produce 100 kg of fuel. That’s nice in a lab, but clearly unaccepable in industry.
Catalyst cost: for example, you better not need appreciable quantities of rare metals (e.g. rhenium, nice catalyst, but 2500 euros per gram).
Recently, much has been written about hydrogenation of CO in its liquid phase (at high pressure, not low temprature). For example here. The catalyst is manganese (price OK) and the “total turnover number” (representing catalyst lifetime) is 12 000, which I’d describe as “good enough to go out of the lab, if cheap enough”. In the summary, I can’t find their batch time. In another study about CO + H2 via manganese, people used a batch time of 8-12 hours. So there is a reactivity issue present, but maybe it can be overcome.