A team of researchers in Kansai is set to begin clinical trials next month to develop medicine to help grow teeth.
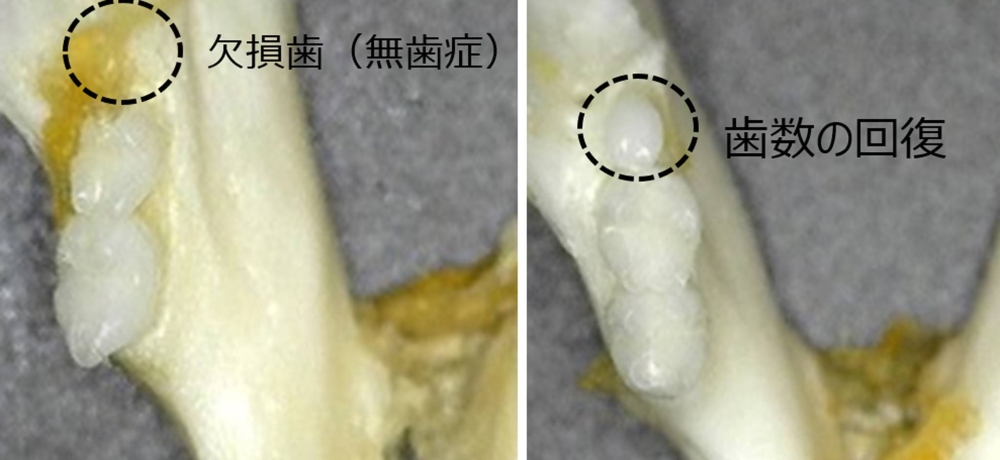
The researchers, including from Kitano Hospital and Kyoto University Hospital, will conduct trials for teething medicine, aimed at treating people with congenital anodontia who are born with few teeth.
To check its safety, the experimental drug will first be administered to adult men who have lost back teeth, before it is tested on children with congenital anodontia. The team aims to put the treatment into practical use in around 2030.


It’s really weird though. Why specify that you’re targeting specifically kids with a disorder when your treatment is being tested on adults, and would work on any adult who has lost teeth for any reason?
Speculating, but probably because kids without any teeth and a genetic basis of that disease would pay for this treatment out of medical coverage, not dental coverage, at least in the US, and getting it approved for specifically that indication is easier, faster, and likely higher profit than the admittedly larger population but smaller insured availability of funds that would be the dental market.
Markets shouldn’t drive drug research, public health benefit should, but shrugs
Good thought, but it looks like they’re a Japanese team conducting trials in Japan, so the US excuse for an insurance system shouldn’t be a factor? IDK
Quick search tells me that they have a national dental plan that covers 70%, but has limits on which procedures materials etc are covered. In other words, they may not cover this procedure if it’s more expensive than a traditional implant.
Additionally, there’s harmonization efforts between Japan and the US FDA; if fully expect they’re running clinical trials in JP with hopes for a later release abroad, and the US is a huge market so they’d still be angling that I’d think.
Because /hobthrob is incorrect. This medication will not and cannot be used to regrow teeth in people that don’t have the genetic disorder “congenital anodontia”. The drug can only help that >1% with the disorder.
That’s not what the article says though